Additional Information
| Specialty | Colorectal, General Surgery, Gastroenterology and Hepatology |
|---|---|
| Manufacturer | |
| Brand |
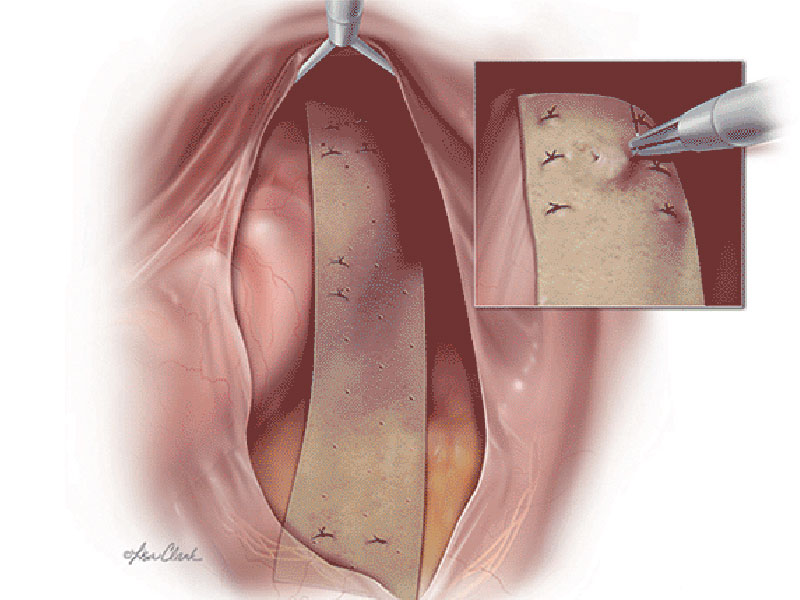
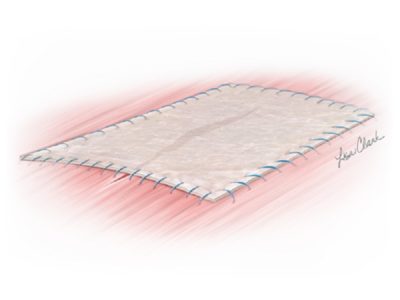
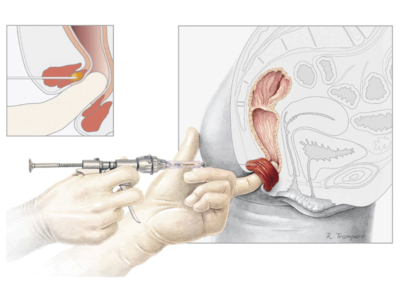
The Biodesign® Rectopexy Graft is intended to support/reinforce soft tissue in surgical procedures for open and laparoscopic repair of rectal prolapse/rectal intussusception. This device is not to be used via a transvaginal approach. The device is supplied sterile and is intended for one time use.
Biodesign grafts encompass the biological properties of porcine small intestinal submucosa (SIS). SIS provides a natural scaffold that allows the body to restore itself through the complex natural process of tissue remodelling. Tissue remodelling involves the recruitment of cells, the renewal of tissue composition, and the reinforcement of structural tissue architecture.¹ As the body heals, SIS is gradually remodelled and integrated into the body, leaving behind organised tissue that provides long-term strength. 2-4
The Biodesign Rectopexy Graft is a non-cross-linked, non-dermis, biologic graft that is completely remodelled into strong, vascularised patient tissue. 2,4,5 As a non-dermis graft, the Biodesign Rectopexy Graft contains no meaningful amounts of elastin.6 Dermis-based biologic grafts contain high amounts of elastin and studies attribute higher rates of failure to higher elastin levels.7,8 The technology behind the Biodesign Rectopexy Graft has been designed to maintain strength throughout the remodelling process, so there is no need for chemical cross-linking.4 Cross-linked grafts have been associated with chronic inflammation and encapsulation.9
The technology behind Biodesign tissue-repair products is supported by more than 1,600 total publications. More than 650 of those describe clinical use and 10 of those have more than five years of follow-up data.
References
1. Turner NJ, Badylak SF. Biologic scaffolds for musculotendinous tissue repair. Eur Cell Mater. 2013;25:130-143.
2. Franklin ME Jr, Trevino JM, Portillo G, Vela I, Glass JL, Gonzalez JJ. The use of porcine small intestinal submucosa as a prosthetic material for laparoscopic hernia repair in infected and potentially contaminated field: Long-term follow-up. Surg Endosc. 2008;22(9):1941-1946.
3. Stelly M, Stelly TC. Histology of CorMatrix bioscaffold 5 years after pericardial closure. Ann Thorac Surg. 2013;96(5):e127-e129.
4. Badylak S, Kokini K, Tullius B, Whitson B. Strength over time of a resorbable bioscaffold for body wall repair in a dog model. J Surg Res. 2001;99(2):282-287.
5. Nihsen ES, Johnson CE, Hiles MC. Bioactivity of small intestinal submucosa and oxidized regenerated cellulose/collagen. Adv Skin Wound Care. 2008;21(10):479-486.
6. Heise RL, Ivanova J, Parekh A, Sacks MS. Generating elastin-rich small intestinal submucosa-based smooth muscle constructs utilizing exogenous growth factors and cyclic mechanical stimulation. Tissue Eng Part A. 2009;15(12):3951-3960..
7. Gupta A, Zahriya K, Mullens PL, Salmassi S, Keshishian A. Ventral herniorrhaphy: experience with two different biosynthetic mesh materials, Surgisis and Alloderm. Hernia. 2006;10(5): 419-425.
8. Kissane NA, Itani KMF. A decade of ventral incisional hernia repairs with biologic acellular dermal matrix: What have we learned? Plast Reconstr Surg. 2012;130(5 Suppl 2):194S-202S.
9. Novitsky YW, Rosen MJ. The biology of biologics: basic science and clinical concepts. Plast Reconstr Surg. 2012;130(5 Suppl 2):9S-17S.
| Specialty | Colorectal, General Surgery, Gastroenterology and Hepatology |
|---|---|
| Manufacturer | |
| Brand |
The information contained within this website is designed and intended for healthcare professionals only.
Endotherapeutics will not be liable for any actions taken in reliance of the information contained within the website. The information contained within the website does not constitute medical advice.
If you are a patient who requires treatment or management of a medical condition, please click No and you will be redirected to Endo Personal Care.