Additional Information
| Specialty | |
|---|---|
| Manufacturer | |
| Brand |
Treatment of male stress urinary incontinence still presents a significant challenge, particularly after radical prostatectomy. The ATOMS System combines a range of features to provide an effective, patient-friendly means of managing all grades of SUI.
There is no surgical reintervention required to adjust the system, the treating physician can simply alter the volume of liquid in the cushion via the access port. Patient-specific adjustment to effect a change in continence status is possible at any time, even in the long-term.¹
ATOMS is made up entirely of components that function hydraulically, thereby saving patients from the difficulties caused by defects which may occur in mechanical components months or years after the implantation.

ATOMS provides effective treatment for mild to severe stress urinary incontinence, even after radiotherapy.
Patients can urinate freely without having to activate a mechanical component. This means the system is also suitable for patients suffering from dementia, or whose cognitive skills may be expected to regress over time. Patients with joint pain (e.g. gout) also benefit from not having to operate the system manually.
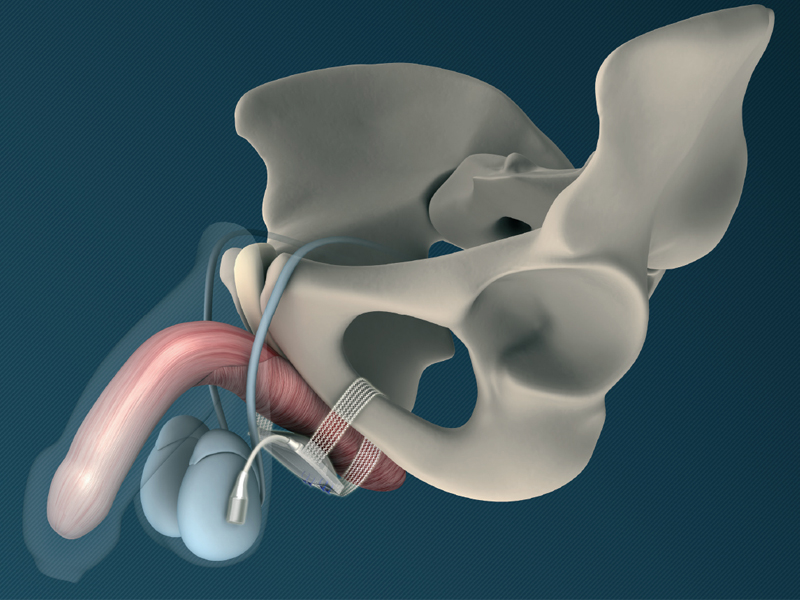
The suburethral substitute sphincter cushion is located in the middle of the mesh fixation tape and is filled via the port-catheter connection after the operation.
This cushion ensures a gentle, evenly-distributed pressure on the bulbospongiosus muscle. Pressure on the urethra is kept to a minimum and the risk of urethral erosion is significantly reduced.
The integrated mesh arms are drawn back around to the middle of the implant to secure the system in place. This eliminates the need for any fasteners or screws, and ensures a symmetrical, 4-point auto fixation.
The results of the latest clinical article demonstrates the long-term safety and efficacy of ATOMS in the treatment of incontinence in men, following invasive prostate treatment.
References
1. Alexander Friedl et al. Long-term outcome of the adjustable transobturator male system (ATOMS): Results of a European multicentre study. BJU International, 2016.
*Cohort of patients who received the latest 3rd Generation ATOMS device with the pre-attached silicone covered scrotal port.
The information contained within this website is designed and intended for healthcare professionals only.
Endotherapeutics will not be liable for any actions taken in reliance of the information contained within the website. The information contained within the website does not constitute medical advice.
If you are a patient who requires treatment or management of a medical condition, please click No and you will be redirected to Endo Personal Care.