Additional Information
| Specialty | Colorectal, General Surgery, Gastroenterology and Hepatology |
|---|---|
| Manufacturer | |
| Brand |
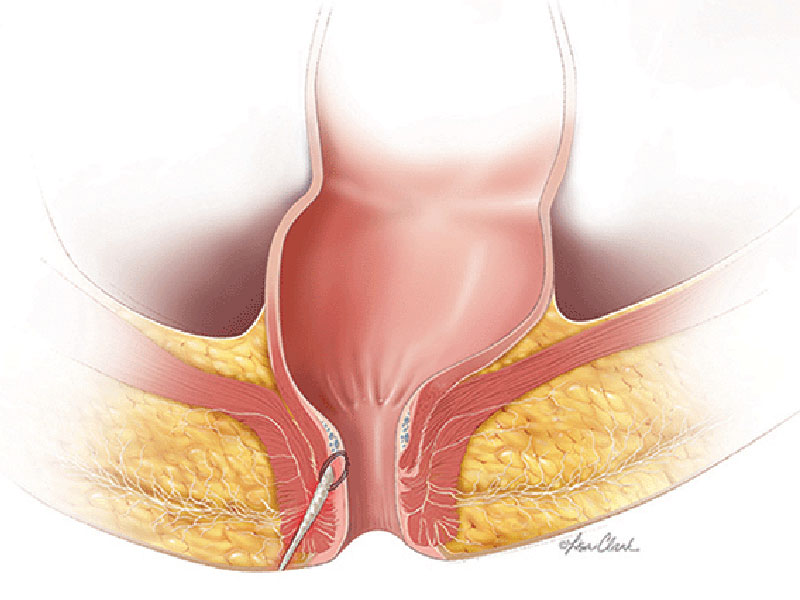
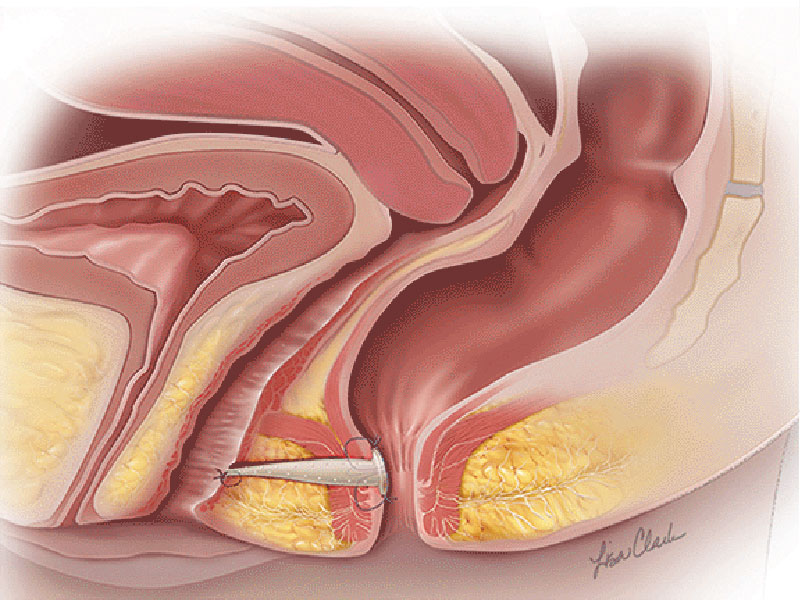
The Biodesign® fistula plugs are a minimally invasive option for sphincter-sparing anorectal or recto-vaginal fistula repair. The Biodesign Fistula plugs have been designed to minimise the risk of postoperative incontinence and can be considered a first choice for treatment.¹ The device is supplied sterile and is intended for one-time use.
Biodesign grafts encompass the biological properties of porcine small intestinal submucosa (SIS). SIS provides a natural scaffold that allows the body to restore itself through the complex natural process of tissue remodelling. Tissue remodelling involves the recruitment of cells, the renewal of tissue composition, and the reinforcement of structural tissue architecture.² As the body heals, SIS is gradually remodelled and integrated into the body, leaving behind organised tissue that provides long-term strength.3-5
The technology behind Biodesign tissue-repair products is supported by more than 1,600 total publications. More than 650 of those describe clinical use and 10 of those have more than five years of follow-up data*.
References
1. van Koperen PJ, Bemelman WA, Gerhards MF, et al. The anal fistula plug treatment compared with the mucosal advancement flap for cryptoglandular high transsphicteric perianal fistula: a double-blinded multicenter randomized trial. Dis Colon Rectum. 2011;54(4):387-393. Note: The name of our product has changed since this trial was published.
2. Turner NJ, Badylak SF. Biologic scaffolds for musculotendinous tissue repair. Eur Cell Mater. 2013;25:130-143.
3. Franklin ME Jr, Trevino JM, Portillo G, Vela I, Glass JL, Gonzalez JJ. The use of porcine small intestinal submucosa as a prosthetic material for laparoscopic hernia repair in infected and potentially contaminated field: Long-term follow-up. Surg Endosc. 2008;22(9):1941-1946.
4. Stelly M, Stelly TC. Histology of CorMatrix bioscaffold 5 years after pericardial closure. Ann Thorac Surg. 2013;96(5):e127-e129.
5. Badylak S, Kokini K, Tullius B, Whitson B. Strength over time of a resorbable bioscaffold for body wall repair in a dog model. J Surg Res. 2001;99(2):282-287.
* Data as of December 2020.
| Specialty | Colorectal, General Surgery, Gastroenterology and Hepatology |
|---|---|
| Manufacturer | |
| Brand |
The information contained within this website is designed and intended for healthcare professionals only.
Endotherapeutics will not be liable for any actions taken in reliance of the information contained within the website. The information contained within the website does not constitute medical advice.
If you are a patient who requires treatment or management of a medical condition, please click No and you will be redirected to Endo Personal Care.